So in that first picture, if you moved the double bond to the amine, the nitrogen would have a negative charge, because it's going to have a double bond, plus 2 hydrogen, plus it's electron pair. nitrogen doesn't like that (octet rule). also the carbon that loses that double bond is going to be positively charged since it will be only bound to 2 carbons and a hydrogen and is missing a valence electron. It's been years since I've done something like that example. But you have a conjugated system.
The electron pair moves from the N to the carbon closest to it to form the double bond, and an electron pair moves from that carbon to the one adjacent to it. I'm a little confused by how they drew it though. but it's been a long time for me and we only went over ortho, para, and meta for one session
here's a decent picture of what I mean
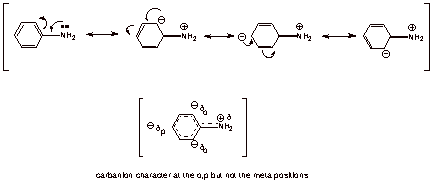
basically the nitrogen has a partial positive charge and all of the carbons have a partial negative if that makes sense